Overview of Proteins, Fat, pH and Water and WHC
In addition to water (≈75%), lean meat also contains about 18.5% protein, 2 to 5% fat, 1.5% non-protein nitrogen substances, and 1.0% each of carbohydrate and inorganic constituents The protein and its dynamic structural arrangement are major factors related to meat's WHC.
In fresh meat, fat is the most quantitatively variable component. As the percentage of fat increases, the percentage water decreases. This relationship is very highly correlated (r = −0.99). There is also a strong correlation between protein and water (r ≈ 0.9. Thus, fat content affects WHC because it is nonpolar (no water holding) and it decreases the relative about of protein available for attracting and holding water.
- Muscle pH: Muscle pH: The pH of meat affects WHC tremendously. During the conversion of muscle to meat, the pH of muscle changes from neutral (pH ≈7.0) to about 5.5 to 5.7, the normal pH of post-rigor muscle. Lactic acid, the byproduct of anaerobic metabolism, is responsible for the decline in pH. Proteins have an electronic charge that changes as the pH changes. The rule of thumb is that the higher the meat pH, the higher the net negative charge on the proteins and the higher the WHC. If proteins become more closely packed because they lack repulsive charges, water is excluded, driving the immobilized water into the free water compartment.
- Isoelectric pH (pHI): All proteins have a characteristic pH where the net electronic charge on the protein is zero (the number of positive and negative charges are equal). This occurs for most meat proteins at pHI 5.1. This pH is called the isoelectric pH where the WHC is the lowest because there is minimal attraction between the proteins and water. Unless a meat product needs to lose water (such as with dried processed products), the meat industry should do everything possible to have meat with a pH above or below the pHI so that the proteins carry an electronic charge to attract water.
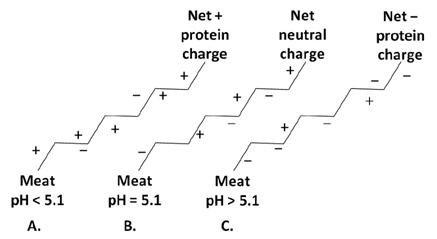
Figure I-1. Net electrostatic charges on protein below, at, and above the isoelectric pH of meat. Meat proteins below the pHI (A) are more positive where as proteins above the pHI (C) are more negatively charged. Both A and C would have greater WHC than meat at pH 5.1 (B).
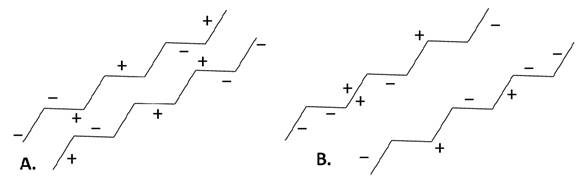
Figure I-2. Effect of electrostatic charge on the spacing between strands of protein. Strands in B have more myofibrillar protein spacing due to a greater net negative charge on the proteins, which results in greater electrostatic charge repulsion compared with the protein in A where there is no net repulsiveness between the protein, resulting in low WHC.
- Dipolar Water: Water (Fig. I-3) also has an electronic charge because the electrons are not uniformly distributed.
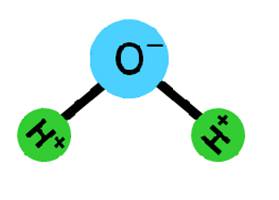
Figure I-3. Sketch of the dipolar nature of water. Note the positive and negative areas within the water molecule that could form electrostatic interactions with meat proteins.
This dipolar electronic structure provides a mechanism where the WHC increases due to the electronic attraction of opposite charges on the protein and the water. Meat's normal pH (5.6) is greater than the protein's pHI, thus meat proteins have some natural attraction for water because the excess of negative charges on the protein are available to attract the positive charged areas of water molecules.