Effect of salt content on chemical and physical properties and implications for organoleptic properties
Salt has an effect on the tenderness, juiciness and flavour of pig-meat products. This section will cover the science behind these effects.
3.3.1 Water-holding capacity
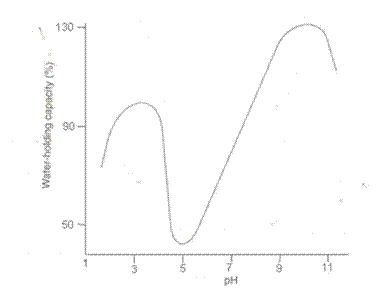
The water-holding capacity of meat can influence its juiciness and tenderness. The classical hypotheses for water-holding capacity in meat are based on electrostatic or osmotic forces.Water-holding capacity is in turn influenced by the electrostatic charge of the proteins within the meat (myosin being the main structural protein). Meat pH influences the extent to which proteins are charged. The muscle proteins reach their isoelectric point (the pH at which they have little or no net charge) as post-mortem acidification progresses. In the pH range between 5.1 and 5.5 (which is close to the pHu) most of the main muscle proteins are at their isoelectric point and the meat fails to attract and hold water, so it releases drip (Gregory, 1998). Figure 1 shows the relationship between pH and water holding capacity.
Figure 3.1 Relationship between pH and water-holding capacity of ground beef (after Hamm)
Salt (NaCl) is highly water soluble. The functions that salt provides in meat mixtures are mainly determined by the dissociated ions Na+ andCl-. When salt is mixed with comminuted meat the Cl- ion increases the negative charge of the proteins. The adsorption of Cl- ions onto the positively charged groups of myosin results in a shift in its isoelectric point to a lower pH, also causing a weakening of the interaction between oppositely charged groups at a pH greater than the isoelectric point. In the presence of salt, part of the insoluble myosin passes into the liquid phase and dissolves, increasing meat swelling and water holding capacity in its dissociated ionized form (H+ OH-). Salt-solubilized myofibrillar proteins form a sticky exudate on the surface of the meat pieces, which binds them together after cooking. This layer forms a matrix of heat-coagulated protein which entraps free water. The increased water-holding capacity of salt-treated meat gives it a higher cooking yield, and greater tenderness and juiciness when the product is consumed (Gregory, 1998; Desmond, 2006; Tarte, 2009). Figure 2 illustrates the process.
Figure 3.2 Mechanism and effect on processed meat products when salt is added
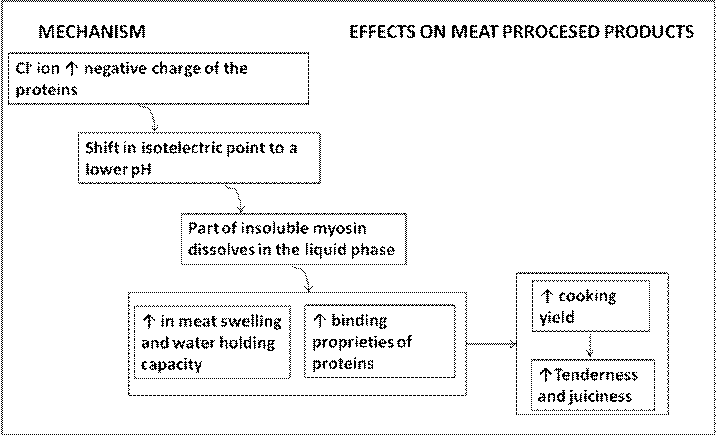
There are alternative explanations on how chloride ions disrupt the myosin molecule and enhance water retention (Puolanne and Halonen, 2010)
Na+ ion, in contrast to Cl- ion, is responsible for the flavour that is delivered by salt. Besides imparting saltiness it increases the intensity of other flavours (Tarté, 2009)
Water-holding capacity and fat retention are closely linked. Myosin is fairly hydrophobic, and when salt is mixed with minced or ground meat, myosin passes to the liquid phase as it dissolves. The presence of a hydrophobic protein in an aqueous phase reduces the repulsion between fat droplets and water in the meat mix. It does this by encapsulating the fat droplets and holding them as an emulsion in the mixture, ultimately increasing tenderness and juiciness (Gregory, 1998).
Although the incorporation of salt enhances protein extraction, it also increases the tendency for oxidative rancidity and discoloration though metamyoglobin formation. An intermediate level of salt gives the benefits of lower cooking loss and greater tenderness without excessive rancidity (Lawrie, 1985). This is illustrated in Table 1.
Table 3.1 Mean values for organoleptic characteristics of flaked pork steaks (Source: Lawrie, 1985)
| Characteristic | Percentage | of salt | ||
| 0 | 0.75 | 1.5 | 2.25 | |
| Rancidity (TBA) | 0.11 | 0.5 | 0.84 | 0.94 |
| Cooking loss (%) | 37.5 | 21 | 14.6 | 13.4 |
| Tenderness (shear:kg) | 1.04 | 0.84 | 0.69 | 0.71 |